
Our Mission
The mission of our lab is to fill the gap between clinical practice and basic science through translational research. Our key research area is cardiovascular tissue engineering using 3D printing/bioprinting technology. First, we aim to use innovative and novel tissue engineering approaches to regenerate the heart using 3D cardiac engineered tissues. The second focus of our lab is patient-specific vascular tissue engineering using 3D printing technology. Our ultimate goal is to create a new history to apply these basic researches into clinical fields to improve patients’ quality of life.
hi

Click to learn more about our wonderful staff and students at Hibino Lab!
hi
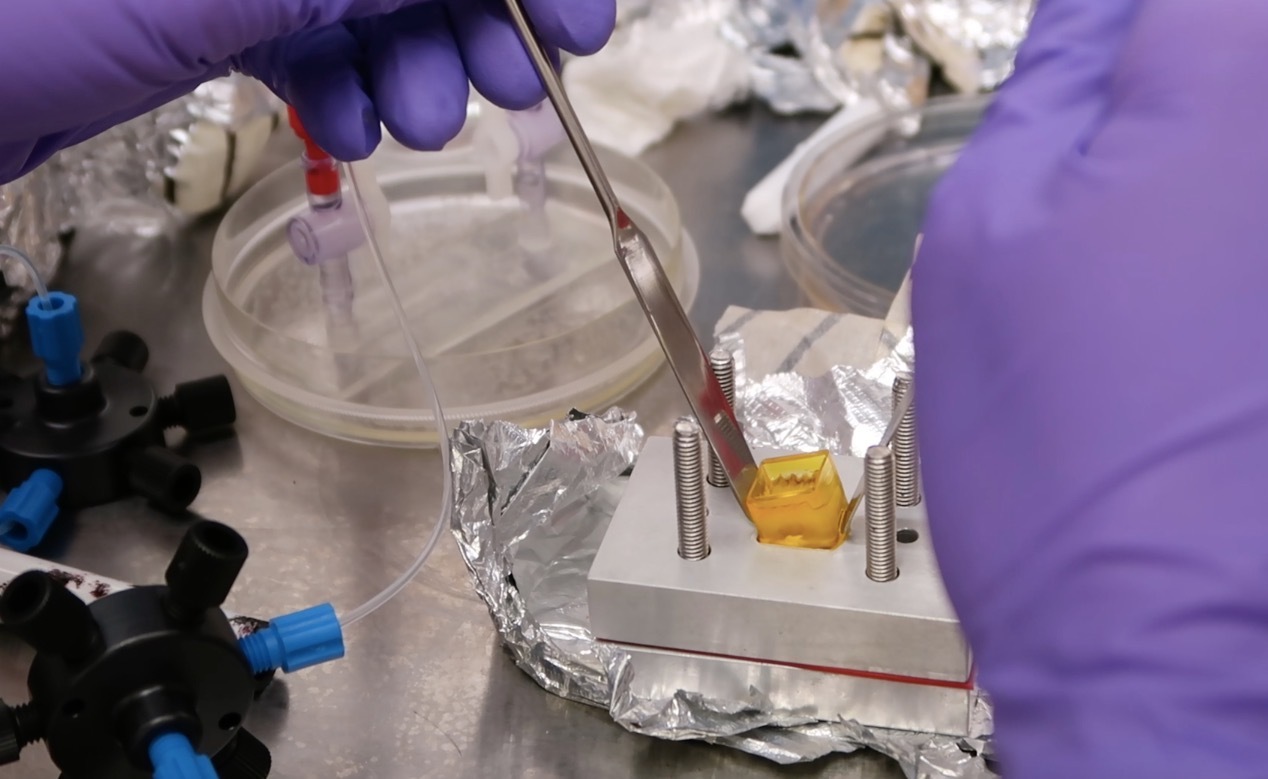
Our Research
Our lab pioneers advancements in cardiac tissue engineering, focusing on vascularization, endothelization, perfusion, and 3D bioprinting. These areas promise to revolutionize regenerative medicine in cardiac tissue engineering.
- Vascularization: We prioritize creating functional blood vessels within engineered heart tissues. Efficient vascularization ensures nutrient delivery, tissue survival, and integration with the host circulatory system.
- Endothelization: We incorporate essential endothelial layers into our tissues to maintain vascular health, prevent clotting, and regulate permeability, mimicking natural blood vessels for long-term stability.
- Perfusion: We simulate blood flow through engineered tissues, enhancing contractile function, nutrient transport, and tissue integration, creating heart tissues resembling native ones.
- 3D Bioprinting: Our lab explores precise 3D bioprinting to replicate native heart tissue’s cellular architecture. This technology allows personalized tissue constructs, potentially improving transplant success rates.
In vascular tissue engineering, we focus on complex vascular reconstruction, offering patient-specific, 3D-printed, tissue-engineered vascular grafts. These grafts optimize hemodynamics, have anti-thrombotic properties, adapt to growth, and reduce neointimal hyperplasia.
Our recent study using a porcine model showed that our patient-specific 3D TEVGs adapt well to growth, maintaining optimal flow dynamics. This research holds promise for vascular reconstruction and cardiovascular care advancement, demonstrating our commitment to a healthier tomorrow.
hey
“I had the fortune of having Dr Hibino as my PhD co-mentor at Johns Hopkins. I was one of the first 3 to join when he first started his lab, so I witnessed the exponential growth of his lab. As a mentor, he is very organized, hands-on, caring, personable and supportive. He has impressive and limitless energy. I strongly recommend him to any prospective undergraduate student, graduate student or postdoctoral fellow, looking for a great research experience.”
Chin Siang Ong, MBBS
hi

